Throat swabs can be used for sampling bacterial contamination monitoring equipment
Bacteria are widespread in the environment, and medical equipment is no exception. In order to reduce the nosocomial infection, need to monitor the pollution of equipment and instruments, but also need to monitor the effectiveness of disinfection and sterilization equipment. Correct sampling is to ensure that the monitoring results an important part. Throat swabs are used to wipe the patient’s throat during routine clinical testing. Throat swab sampling easy to use, with fewer errors, we will pharyngeal swab for monitoring equipment sampling.
material:
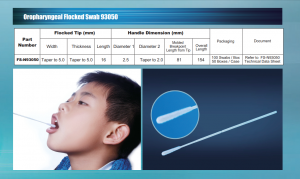
Medico throat swab is a sterile product, radiation disinfection, shelf life of 2 साल. The appearance of small plastic rods about 10 सेमी, one is a nylon flocking fiber swab. Mainly used for clinical patients pharyngeal samples collected, but also for skin exudate, घाव, epidermal specimens collected detection.
method:
(1) bacterial swab test sampling method
Dry throat swab is used directly for rubbing, wet throat swab is used in the broth tube after rubbed. Sampling swab placed in the broth after full elution, the broth tube was placed in 37 Tuen training 24h. 37 Tuntang transfer plate, 24h culture. Take a single colony micro-enzyme, oxidase, coagulase experiments, while doing Gram stain, distinguish Gram-positive bacteria (G) and Gram-negative bacteria (G).
(2) Bacterial identification
Take a single colony of sterile cotton swabs to release 1.8 एमएल 0. 45% NaCI sterile saline, make it into a bacterial suspension, the preparation of the concentration of No. 1 Macrobrachium (G bacteria) या 0. 5 Microbial cells (G bacteria) bacterial suspension inoculated with GN I card or GPI card. After inoculation of the card all released AN S system analysis.
bacterial swab test conclusion
Sampling can be used throat swabs or throat swabs can be used for bacterial contamination monitoring equipment sampling, this method of sampling is simple and reliable, but only for qualitative, can not meet the quantitative testing requirements. During the banquet, many of them are qualitatively monitored, indicating that aseptic or bacterial type can be determined.

















